Key stages
The bulk of the data will be collected via regular surveys of the children’s parents, in the form of telephone interviews and postal or Internet questionnaires.
The different stages of the study are carried out either by expert interviewers or by professionals who come into contact with the children in the course of their work, be they healthcare staff conducting the initial observations in the maternity units, physicians collecting more technical information about growth, innoculations, psychomotor development and so on, or teachers assessing the children’s learning.
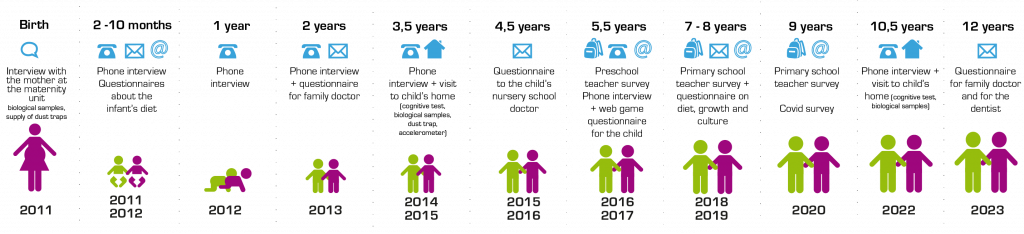
We first met the Elfe children during a home visit, when they were 3½ years old. As they grow older, they will become increasingly closely involved in the study (e.g. fun questionnaires to complete over the Internet at age 5‑6 years, drawing of a man at 7½ years).
At key stages in their development, we will collect biological samples from some of the families, and environmental measures will be carried out in selected homes. When the children turn 8, there are plans to collect the milk teeth they have lost, as these teeth contain traces of many different kinds of environmental exposure.
Fathers’ participation
One of the novel features of the Elfe study is the extent of fathers’ participation, as their role and involvement in the family circle have changed considerably over the past few decades. In the Elfe study, fathers are regularly contacted in the course of the follow up, and we also take account of specific circumstances such as parental separation. Fathers may, for example, be asked about the sharing of childcare and household tasks, housing conditions, the family’s living conditions, their relationship with their child, and their health and lifestyle. Fathers can also be identified as primary caregivers instead of mothers.
Health insurance data
Data from the various health insurance schemes operated by France’s social security system are collated in a national database (SNIIRAM). This database was originally created to improve the way that health insurance and healthcare policies are managed, as well as to enhance care provision. SNIIRAM data on the treatment and hospital care received by the mothers during their pregnancy, as well as on their child’s subsequent healthcare consumption, may be used to enrich Elfe study data. The aim is to lend greater detail to the health information provided by parents, especially regarding any drugs that are prescribed, without having to ask them directly. These invaluable data will add to what we already know about the health of the mothers during pregnancy and that of their children, as well as the medication they receive. Setting up the procedure whereby SNIIRAM could pass this information on to the Elfe unit was a very lengthy legal and administrative process, but it will finally begin in 2019 for those mothers and children for whom consent forms were signed at the maternity units ‑ unless the parents have since made their opposition known. Processing will comply with stringent security guidelines laid down by the French Data Protection Authority (CNIL) guaranteeing the protection and responsible use of these data. Access to the raw health insurance data will be limited to the member of the Elfe team in charge of the SNIIRAM database. The data made available to researchers will be preprocessed to ensure participants’ anonymity. All research projects using these data will be listed in a specific section on the Research Projects page. It should be noted that all persons are free to withdraw their consent to this data linkage, by sending a request in writing to Dr Marie-Aline Charles (Elfe - Ined, 9 cours des humanités, CS 50004, 93322 Aubervilliers Cedex).

